
What is Meant by Evergreening of Patents?
The term evergreening is used to refer to perceived attempts to extend the ordinary duration for which a patent is protected by deploying different means. The proprietor of the invention often tries to secure multiple patents covering different aspects of the same product. Also, much often in the pharmaceutical sector, improved versions of the patented inventions are attempted to be secured to achieve evergreening of a single patented invention. Evergreening is not essentially permissible; however, in South Africa, this problem is prevalent due to an incomplete understanding of the Patent System. As per the TRIPS Agreement, countries may adopt strict patentability criteria to overcome this issue through limiting the grant of patents on new uses or minor modifications of the existing medicinal products, as a result of which, countries like Argentina, India, Philippines have adopted a strict and rigid approach.
Position on Evergreening of Patents in South Africa
The Patent Act in South Africa does not say anything about evergreening. But, patentees often rely on Section 39 of the Act, which states that an improvement of an existent patent can be obtained even if the said improvement is obvious to the main invention. However, the argument does not hold strong since the section provides that the term of such improvement cannot extend beyond the timeline of the original/main invention. The same is termed as the patent of addition.
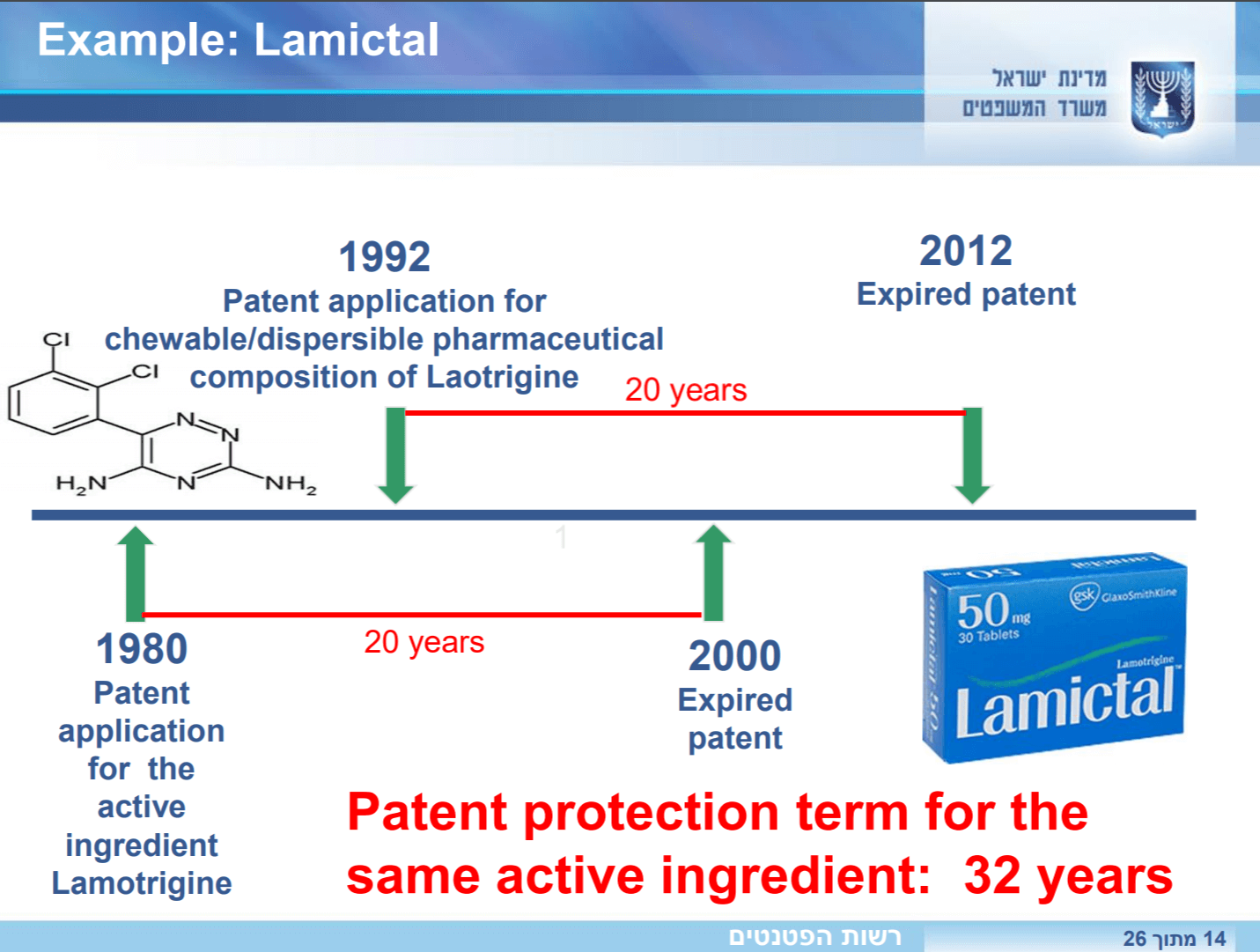
However, the general practice followed in South Africa favors inventors in this aspect. There is no substantive screening of patents since only a thorough check of documents is carried out. Therefore, the only time a patent is adjudged on its merits is if the patent is challenged in court, which is something that only really happens as a response to a claim of infringement. Consider the fact where a generic company contests the validity of a patent due to evergreening or due to lack of novelty since the patent is based on an incremental form of the invention that preexisted; it is most likely that the pharmaceutical company will manage to receive an interim interdict because of the ‘balance of convenience,’ which favors it, and therefore, the issue of the validity of the patent will be put-off for another day that could take up to another year’s time. It simply translates to the fact that irrespective of the merits of the new patented invention, the pharmaceutical company can always buy a few years of extra protection in its favor.
It majorly prevents generics from doing what they do best. It withholds them from exploiting the rights, which fall into the public domain after the rights in a particular patent are extinguished. The original patented invention is often patented again by projecting a different manufacturing process or a dissolution profile, which is contrary to public benefit.
It is because of this reason that certain parties and elements of the society, including the Treatment Action Campaign, are asking for a proper reformation of the existent patent system in South Africa in favor of distribution of cheaper drugs and manufacturing of generics to regulate the pharmaceutical sector after the term of the patent is done away with. Such reform would call for an amendment into requisite clauses of the Patent Act to include clauses similar to those found in Section 3 of the Indian Patent Act, which enables a generic-friendly approach and creates a fine line balance between private entities and generic manufacturers.
Major Issues in the South African Patent System
The following are a few notable issues that intervene in bringing a fruitful reform to prevent the evergreening of patents in South Africa:
- South Africa’s Depository System: As has been noted above, there is a depositary system wherein the Patent Applications, which are submitted for admission, are subjected to a rudimentary check of documentary requirements only. Therefore, the Intellectual Property Consultative Framework (IPCF) in the year 2016 proposed a new framework to replace the existing system. It called for a Substantive Search and Examination (SSE) system. The move was backed by other channels as well, including Fix the Patent Laws Coalition, which strives to address the issues of public and affordable access to medicine in the country. It is opined that such a system of substantive search would ensure that patents are only granted on real innovations, thus limiting the grant of weak patents. The reason for the grant of repetitive patent and soft/weak patents is the reason why approximately 40% of the patents admitted by South Africa are rejected on scrutiny by the European and US Patent Office.
- Logistic and Financial Incapacity: Bringing a reformative change and introducing a new framework to adopt the Substantive Search and Examination system (SSE) would require qualified patent examiners who are well equipped with the cutting edge of technology. This could be an expensive affair, and finding such professionals in sizable numbers is also questionable. The South African Patent Office is, in today’s date, only barely able to meet its commitments to the basic depositary system. Also, it has had a long-standing service delivery shortfall relating to its filing service provider, with the result that patents open to public inspection cannot be accessed. Therefore, the recordal of transactions of patents are falling behind.
- Barriers to Assess when the Patent actually Expires: The following are the barriers that prevent the determination of actual patent tenure:
- Insufficient public information and the complexity of patent documents contribute to the lack of transparency in the current system. Also, the online database does not always contain full-textual information, which can be misleading in nature.
- Non-disclosure of International Non-proprietary Names (INNs) in patent applications results in the use of diagrams of chemical structures, which makes it extremely difficult to point out all the relevant patents covering a particular medical invention.
- Markush-type claims are often found in patent literature in South Africa that constitute up to 59% of the patents filed in total, which allows patentees to mask the actual invention while making. It is extremely difficult to conduct prior-art searches.
- Obscure manner of drafting often makes it difficult for competitors to predict whether or not a patent would be granted.
Therefore, sustaining the current system is a seemingly glaring problem because of which the introduction of a new system may or may not be the right choice as it may backfire.
Conclusion: Why Put an End to Evergreening?
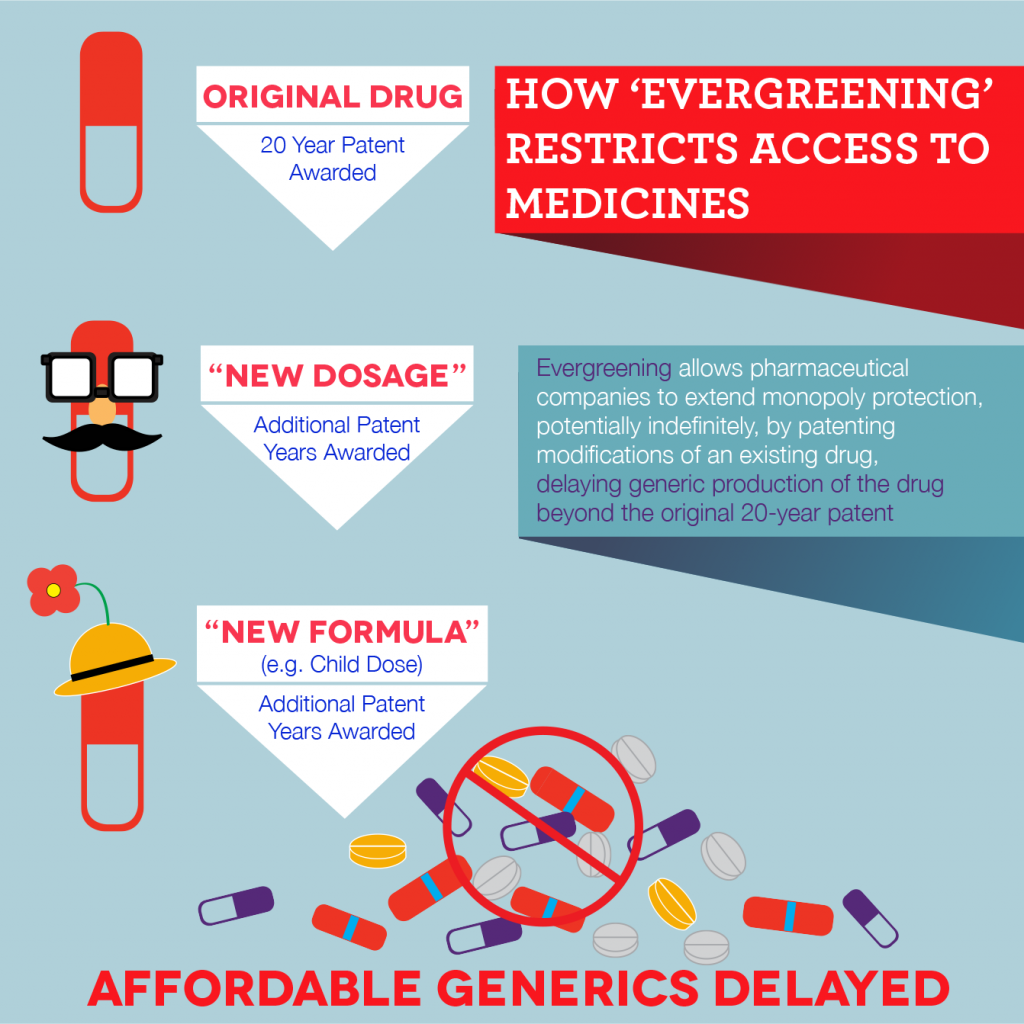
The proliferation of secondary patents introduces a culture of uncertainty and leads to the fear of untimely Patent Infringement, which ultimately discourages generic drug producers from distributing affordable health care products. Let’s consider the case of the cancer treatment drug, imatinib that is originally held by Novartis in South Africa, which was due to expire in April 2013. However, a perpetual patent filed based on new use has extended the patent timeline on imatinib till 2022. Only Cipla managed to reach an agreement with Novartis for the drug by ousting other would-be competitors. As a result, Cipla makes the drug at a price that is 91% cheaper than that sold in South Africa, which would prevent others from entering the market. This ultimately promotes unhealthy competition and works against public health interests.
Therefore, what South Africa needs is a reform in its practices since it actively/passively permits evergreening. It has weak requirements concerning patentability, does not provide for pre and post-grant opposition procedures, and has a very cumbersome provision for the grant of compulsory license. It is time that it adopts TRIPS flexibilities and makes amends to its Patent Laws.

