
After a lot of intense negotiations and debates between the first-world countries and the third-world countries, the World Trade Organization (WTO) Committee on Intellectual Property Rights (IPRs) decided to extend the waiver allowing Least developed Countries (LDCs) from being excused from their obligation of applying and enforcing IPRs on pharmaceutical products and medicines, in general, until 2033. The initial period of exemption from the obligation to ensure compliance with the TRIPS Agreement as per Article 66.2 of the Agreement was a total of 10 years, which has now been extended up to 1st January 2033, until it is renewed again. It means that LDCs are not liable to grant patents to pharma products; neither is there any prohibition on reverse engineering, which is why low-cost generic pharma companies can freely operate in favor of public health.
Number of Extensions Until Now
There have been several occasions where LDCs have requested extensions concerning the application of the transition period. The timeline is projected as follows:
- The most initial deadline for the transition of LDCs into full compliance with the obligations narrated in the TRIPS Agreement was 1st January 2006 that the TRIPS Council provided for. It stated that the Council “shall, upon duly motivated request by an LDC Member, accord extensions of this period.”
- Thereafter, the first extension was particularly related to pharmaceutical patents, which lasted until 1st January 2016. The subsequent extension was approved by the TRIPS Council on 29th November 2005, which meant that LDCs did not have to apply the provisions under the TRIPS Agreement, in general, until 1st July 2013. Again, this period was extended to 1st July 2021 by a TRIPS Council decision taken on 11th June 2013.
- Lastly, the Doha waiver that specifically addressed pharmaceutical patents was further extended until 1st January 2033 based on a request from a group of LDCs.
Purpose of Extending the LDC Transition Period
While the extension of the transition period is facilitated to speed up the process of graduating from the LDC category to the category of a developing nation as well as to create a viable technological base to promote TRIPS measures, many hold that such extension would simply result in postponing the implementation of TRIPS and would waste the time that could be utilized to improve the current situation.
The extensions granted to the LDCs is based on Article 66.2, which aims to provide them with not merely requisite time to comply, but is also meant to aid LDCs to curate their national policies/laws/rules and economies to ensure that the eventual implementation of the TRIPS Agreement will promote rather than undermine their social, economic, and environmental wellbeing.
Need for Technical and Financial Cooperation
It is pertinent to note that a mere extension and the intent of such an extension would not suffice the need of the hour. It is imperative to extend technical and financial cooperation. In the decision made by the Council, no such link to support the development or provide for the wide dissemination of technology can be seen. Although the reference to Article 66.2 is often made, which speaks about developed countries to “provide incentives to enterprises and institutions in their territories for the purpose of promoting and encouraging technology transfer to [LDCs] in order to enable them to create a sound and viable technological base.” However, this is a vague provision as both the nature and the quantity of the incentives are not indicated. Since the same is ambiguous, it also translates to the fact that the obligation therein is also uncertain – when it comes to its application. Article 67 is also equally ambiguous in this aspect since it asks technical and financial assistance to be extended in favor of LDC.
How can LDCs Move from this Category to the Category of Developing Nations?
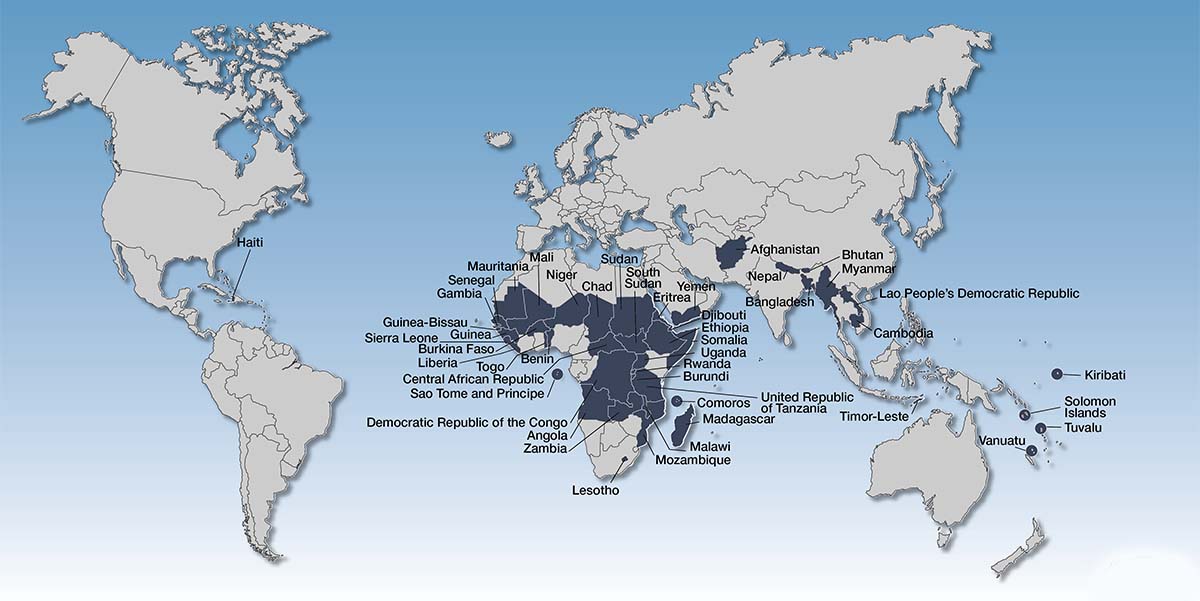
As of now, there are a total of 33 African LDCs, 9 Asian LDCs, 1 in the Caribbean, and 4 in the Pacific. Furthermore, a total of 3 eligible countries declined to become LDCs, which includes Ghana, Papua New Guinea, and Zimbabwe. Since its inception, only a few countries have managed to graduate and transition from the LDC status. These include Botswana on 19th December 1994, Cape Verde on 20th December 2007, Maldives on 1st January 2011, and Samoa on 1st January 2014.
The basis on which an LDC status is granted to a country, which makes it exempted from pursuing obligation in the TRIPS Agreement, is mentioned hereunder:
- Low Income – It isbased on Gross National Income (GNI) per capita (a three-year average), with thresholds of US$ 905 for cases of addition to the list.
- Human Assets Weakness –It is based on a composite index (the Human Assets Index, HAI) that consists of indicators on nutrition, health, school enrolment, and literacy.
- Economic Vulnerability – It isbased on a composite index (the Economic Vulnerability Index, EVI) that includes indicators on natural shocks, trade shocks, exposure to shocks, economic smallness, and economic remoteness.
Therefore, the key factors that could help in the transition are as follows:
- Rapid progress in industrial development;
- Commercialization of research or development (R&D) of strong and creative industries; and
- Adoption of a national Intellectual Property (IP) policy in consultation with different stakeholders while integrating national developmental goals inclusive of public health, unemployment and poverty reduction, climate change mitigation, and adaptation to promote innovation.
Impact of the Extension on the Pharmaceutical Sector
India is one of the largest generic producers of drugs. Although it might not be impacted directly through any alteration or modification in the legislative aspect, it most certainly has been affected in terms of marketing and distribution of drugs. For example, an African based drug manufacturing company brought the Indian pharma major and manufacturer of the world’s largest generic drugs, Cipla Ltd, on its board. Since then, Cipla has increased its stake in the firm to 51 percent. Therefore, LDCs will continue to import generic drugs until they are capable of achieving adequate manufacturing techniques and facilities domestically, which means profits in literal terms for all importing countries alike.
 Therefore, it means that India and other generic manufacturing nations will continue to play a significant role in contributing to the pharmaceutical market, which will eventually impact the business and market in India and other such countries.
Therefore, it means that India and other generic manufacturing nations will continue to play a significant role in contributing to the pharmaceutical market, which will eventually impact the business and market in India and other such countries.
In the long run, LDCs and developing countries have effectively used the transition periods to match-up and improve access to treatment for life-threatening diseases like HIV and its co-infections by importing or manufacturing lower-cost generic medicines. However, medicines and drugs like Sofosbuvir, which are used to treat chronic Hepatitis C, remain a grave challenge among such nations because of higher prices imposed, which may extend up to costing as much as $84,000 for a 12-week course in developed countries.
In the race against killer diseases like malaria and HIV, the world’s poor countries are largely dependent on generic drugs, coming from generic manufacturers such as India and Brazil. Bangladesh, for instance, has been making use of its LDC status and has launched its version of Sofosbuvir, costing as little as $900 for the 12-week course.
Concluding Remarks
Although such extensions can be best utilized in favor of meeting public health requirements, LDCs have not utilized these transition periods even after making subsequent requests of claiming extensions. Also, there have been no such studies conducted to determine the action taken and the improvement of the situation in the LDCs. To ensure that transition periods are utilized in the best possible manner, the technical and financial assistance should be extended to government agencies, as well as the R&D department of colleges and universities. Also, constant feedback and appraisal from such countries should be gathered to ensure the direction of growth and development over the years gone by, as well as the effort to be put in terms of times yet to come.

